Check Out Your Online Account
Accurate Screw Machine Products
Download the ASM Catalog or Credit Application
Accurate Screw Machine specializes in just-in-time delivery of high-quality electronic hardware and fasteners. Browse our catalog for details on our products and services, including everything from bushings and captive screws to locking fasteners, handles, and washers
CAGE Code: 51506

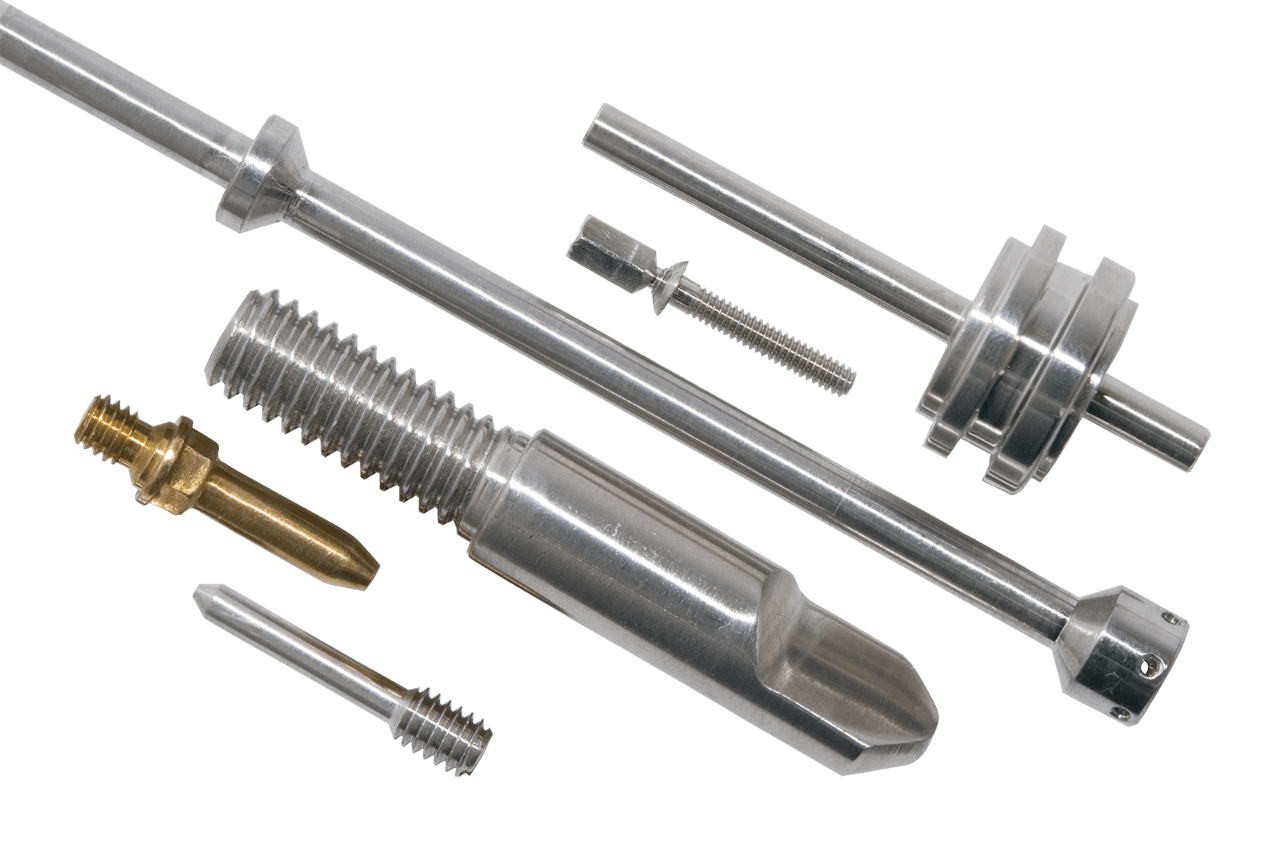
Prototype to Production Manufacturing
ASM offers diverse manufacturing capabilities, including everything from prototyping to full production capabilities. We manufacture components to metric and standard sizes, with a large selection of heads, threads, lengths, IDs, and ODs.
We also offer an extensive list of available finishes and plating options. Review the list HERE.
Need more support? Our engineers can supply design for manufacturability support and custom CADs for modified parts after you request a quote. Get in touch with us for details.

Ask an Engineer
Custom Electronic Hardware & Fasteners
Custom Machining
Speed and precision matter. As one of the top specialty fastener manufacturers in the United States, ASM leads the way in precision machining and custom part manufacturing. Our precision fastener components are tailored to your specific needs, including custom screws. Get in touch with us to request a quote for your custom parts.